According to news released by Changchun Institute of Applied Chemsitry (CIAC), subnanometer-sized copper nanoclusters were prepared by a one-pot procedure based on wet chemical reduction.The scientists demonstrates that the structural characteristics of the 2-mercapto-5-n-propylpyrimidine-protected nanoclusters, Cun (n≤8), were determined by mass spectrometry. The Cu nanoclusters displayed apparent luminescence, with dual emissions at 425 and 593 nm, with quantum yields of 3.5 and 0.9%, respectively, and high electrocatalytic activity in the electroreduction of oxygen.
In their research, Dr. CHEN Wei and colleagues list that this is the first time that stable copper nanoclusters with less than eight atoms in the core were synthesized. The clusters exhibited interesting dual luminescence, and thus might be exploited as a novel fluorophore. Such low-cost electrocatalyst might serve as effective cathode catalysts in alkaline fuel cells. Ongoing work is focused on the investigation of the crystal structure and size-controlled synthesis of Cu nanoclusters and the size-dependent electrocatalytic activity for oxygen reduction, and results will be reported on due course.
The research results have been published in J. Am. Chem. Soc. 2011, 133, 2060–2063. The work was supported by the National Natural Science Foundation of China (No. 21043013) and CIAC (W.C.). S.C. acknowledges support by the National Science Foundation (CHE-0718170 and CHE-1012258).
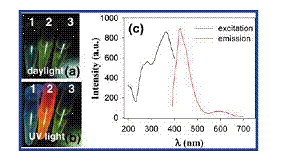
Figure. Photographs of the samples under (a) daylight and (b) UV light: (1) empty tube; (2) Cu clusters in CHCl3; (3) MPP in CHCl3. (c) Excitation (black curve, λem = 425 nm) and emission (red curve, λex = 364 nm) spectra of the copper nanoclusters in CHCl3. |
