|
|
Research Divisions
Research Progress
Achievements
|
|
|
| Metallo-Supramolecular Complex Enantioselectively Targeting Amyloid for Alzheimer’s Disease Treatment |
 |
Text Size: A A A |
|
Alzheimer’s disease (AD), the most prevalent age-related dementia, affects more than 30 million people worldwide. The origin of neurotoxicity is closely linked to the amyloid (Aβ) aggregation in AD pathogenesis. Therefore, inhibition of Aβ aggregation and understanding the mechanisms have attracted much attention. Although considerable achievements have been made, the complex interactions involved in chiral Aβ aggregation, dynamics and morphology are difficult to address using conventional small molecules. Therefore,highly selective recognition of the chiral Aβ supramolecular structures may be a key for understanding of Aβ neurotoxicity and discovering new therapeutic agents. Emerging evidence suggests that stabilization of Aβ helical motif may be a suitable target for inhibiting Aβ aggregation. Certain self-assembling chiral multimetallic coordination complexes, named helicates, can have similar size to α-helical peptides, particularly in terms of their diameter and charge, and this can lead to specific target-binding affinity with biomolecules. Therefore, chiral supramolecules which are capable of binding the α-helical form may selectively inhibit Aβ aggregation. Dr. Xiaogang Qu (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences) and his collaborators have reported that chiral helicates with symmetric rigid ligands AB–BA (Fig. A) can be potential therapeutic agents for targeting Aβ (Chem. Sci., 2012, 3, 3145–3153; J. Am. Chem. Soc. 2014, 136, 11655−11663). However, this symmetric system offers little scope to adjust the structure to improve the selectivity and interaction with Aβ or to create a library for screening Aβ aggregation inhibitors due to the high symmetry and modest functionality of the system. In their current work (Guan, YJ et al., Sci. Adv. 2018, 4, eaao6718), by taking a new strategy whereby directional ligands AB–CD are used to synthesize optically pure head-to-head-to-tail (HHT) asymmetric constitutions in the absence of head-to-head-to-head (HHH) isomers (Fig. B) and strategically prepared a highly stereoselective self-assembly of diverse, Qu and his collaborators found these metallohelices with the HHT structure to enantioselectively inhibit Aβ aggregation by using a live cell-based high-throughput screening system (Angew. Chem. Int. Ed. 2011, 50, 4184–4188; Nat. Commun. 2014, 5, 3422). These chiral metallohelices have amphipathic topology and high, structure-dependent, selective biological activity. The Λ- enantiomer shows specifically target the central hydrophobic α/β discordant stretch and shows much stronger effect on inhibition of Aβ42 aggregation than the Δ-enantiomers, evidenced by multiple biophysical and biochemical techniques, NMR spectroscopy, FTIR and molecular simulations studies. More intriguingly, Aβ can cause signaling amplification that inactivates superoxide dismutase (SOD) and generates additional free radicals. In this work, they found that the metallohelicate has SOD activity as an artificial enzyme to further deplete cytotoxic superoxide (O2−) radicals. Further studies demonstrate that these metallohelices can cross the blood-brain barrier (BBB) and block Aβ-mediated cellular toxicity. In vivo studies prove that these metallohelices extend the life span of AD model C. elegans CL2006 strain by attenuating Aβ-induced toxicity. This work indicates that these new generations of asymmetric chiral supramolecular complexes are novel potent Aβ inhibitors for AD treatment. 
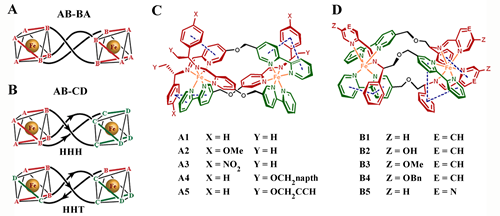
Figure (A) Symmetric metallohelices based on rigid ditopic bidentate ligands AB-BA give D3-symmetric enantiomers [Fe2(AB-BA)3]. (B) The directional ligands AB-CD lead to C1-symmetric HHH and HHT “triplex” architectures. (C and D) Metallohelices self-assembled from various components of a range of functionalized helices with HHT structure. Me, methyl; napth, naphthyl; Bn, benzyl. Xiaogang Qu(曲晓刚), PhD, FRSC Professor of Chemistry, Associate Editor, RSC Journal of Materials Chemistry B, Laboratory of Chemical Biology and State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, China Tel: 86-431-8526-2656(O);
Fax: 86-431-8526-2656 E-mail: xqu@ciac.ac.cn Website: http://yjsb.ciac.jl.cn/daoshi_read.php?brow=47 |
|
|
