With the rapid development and expanding demand of novel electric transportation and grid-scale power storage, traditional lithium-ion (Li-ion) batteries cannot satisfy the demand of such large-scale electricity consumption. Rechargeable aprotic lithium-oxygen (Li-O2) batteries have become burgeoning candidates because of their ultrahigh theoretical energy density of 3,500 Wh kg?1, which is about 10-fold higher than that of Li-ion batteries. Lithium metal as anode is one of the key factors in obtaining such high specific capacity thanks to its lowest electrochemical potential (?3.040 V versus standard hydrogen electrode) and high theoretical capacity (3,860 mAh g?1).
However, the use of Li metal anode inevitably triggers serious safety issues because the dendrite growth of Li will pierce the separator and give rise to a short-circuit fire. Furthermore, in sharp contrast to other Li metal-based batteries such as Li metal-ion battery and Li-S battery, the semi-open nature as well as the oxidizing environment of Li-O2 batteries would result in more severe parasitic side reactions between Li anode and O2 (dissolved in electrolyte), H2O (generated from the decomposition of electrolyte), and other contaminants (e.g., discharge intermediate), which significantly hinders the development of Li-O2 batteries. Therefore, it is vital and urgent to open up a path to effectively protect the Li metal anode of Li-O2 batteries.
Recently, a research team led by ZHANG Xinbo from the Changchun Institute of Applied Chemistry (CIAC), Chinese Academy of Sciences developed an electrolyte regulation strategy by in situ coupling of CF3SO3? on the hydrophobic silica colloidal particles via electrostatic interactions to prevent lithium dendrite growth and corrosion. Their findings were published in Matter.
They found that this strategy can simply couple the anion with nanosilica via electrostatic interaction to avoid the formation of strong electrical field during lithium deposition process. The higher Li+ transference number, uniform heterogeneous nucleation sites and solid-like rheological properties can synergetically lead uniform lithium deposition.
Besides, low diffusion coefficient of 10 wt% HSCE and hydrophobic property of silica lead to 980 times better anticorrosion effect, which greatly improve the lithium corrosion condition in Li-O2 batteries.
Moreover, with the assistance of 10 wt% HSCE, a stable and long life electrochemical performance has been obtained in Li-O2 batteries.
“We believe that this comprehensive and effective protection strategy can spark more inspiration in electrolyte regulation method to obtain better electrochemical performance.” said ZHANG.
Meanwhile, this study can also provide an effective electrolyte regulation strategy to solve the daunting dendrite and corrosion issues in alkali-O2 batteries and alkali-air batteries. These batteries have the potential to achieve good electrochemical performance in practical applications, and adopt collaborative methods to release the great potential of alkali metal anode.
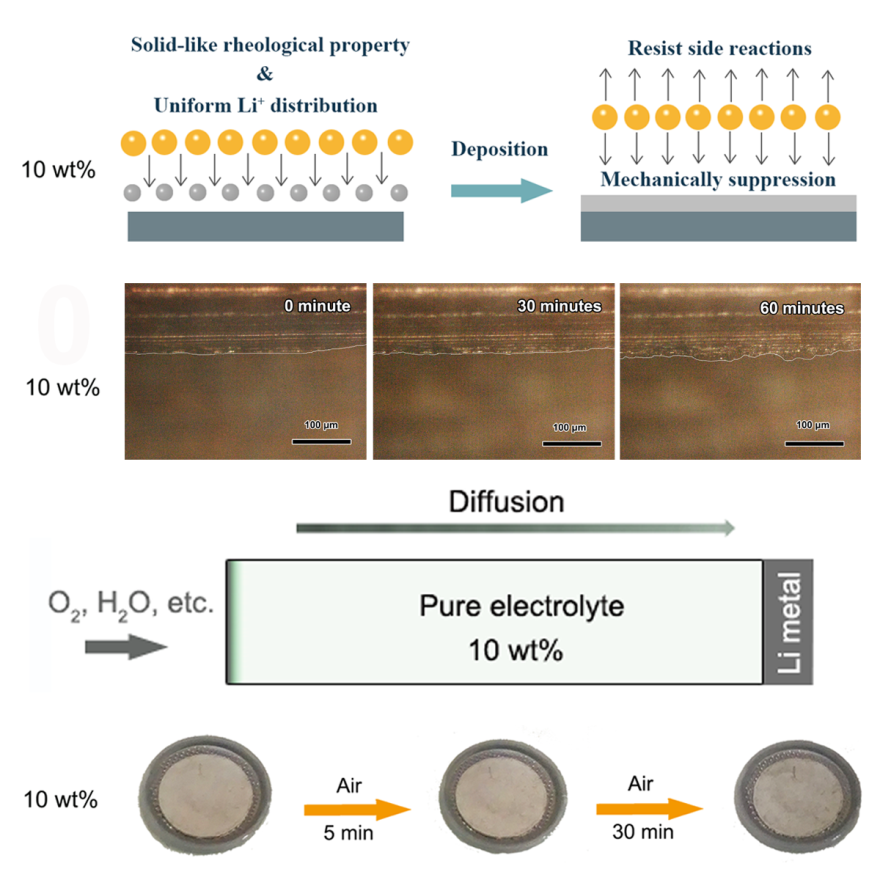
The schematic graph and experimental data of the lithium dendrite prevention effect of 10 wt % HSCE; The schematic graph and experimental data of the anticorrosion effect of 10 wt % HSCE. (Image By ZHANG Xinbo)
1Contact:
Xinbo Zhang
Email: xbzhang@ciac.ac.cn
Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
