A trade-off exists between hardness and stretchability of almost all polymeric materials like plastics, elastomers, and fibers, which limits the toughness of the polymeric materials determined by the stress-strain curve. One practical approach to improve the toughness of polymeric materials is introducing double networks with one network to maintain the network integrity and the other interpenetrated network to sacrifice and dissipate the energy. However, the disadvantage of this approach is that the sample cannot recover to its original state after breaking the covalent bonds of the sacrificial network. Prof. CHEN Quan from the Changchun Institute of Applied Chemistry (CIAC) of the Chinese Academy of Sciences and his colleagues recently explored an effective protocol for toughening vitrimers based on dioxaborolane metathesis through introducing a reversible secondary interaction and revealed the underlying molecular mechanism of this protocol. This work was published in Polymer Science & Technology (DOI: 10.1021/polymscitech.4c00008) on November 8, 2024. In this study, they copolymerized hexyl methacrylate with hydrogen-bonding n-isopropyl methacrylamide and vitrimeric cross-linkers to prepare dual-cross-linked networks. During the tensile tests, the energy dissipation was greatly enhanced by increasing the density of hydrogen bonds, enabling the initial modulus to increase from ∼1 MPa, which is typical for an elastomer, to 300 MPa, which is closer to the glass. Nevertheless, the breakup of the hydrogen bonds during the elongation significantly dissipated the energy, leading to the softening of the materials, thereby facilitating the stretch to high strain. The softening process, seen as an overshoot in stress-strain curve, was well captured by the Dobrynin model modified by Konkolewicz and co-workers upon including a strain rate-dependent element. The deviation at a high content of hydrogen bonds, where the distance between hydrogen bonds becomes smaller than the Kuhn length, was attributed to the coupled motion of the hydrogen bonds.
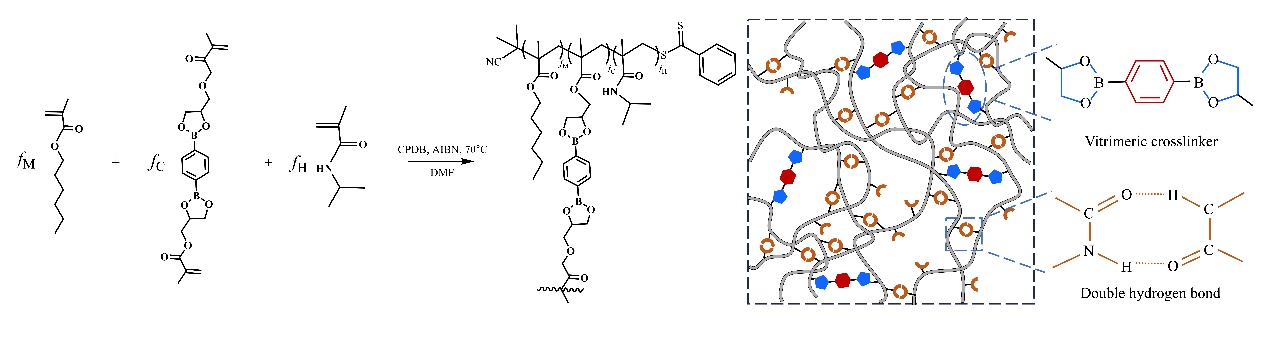
Structure of the Dual-Cross-Linked Network
|
