For a long time, ketamine's antidepressant mechanism has been explained within the theoretical framework of NMDA receptor antagonism—that is, by blocking NMDA receptors, it triggers downstream glutamatergic synaptic enhancement and neuroplasticity reconstruction. However, with the continuous accumulation of clinical and preclinical evidence, the limitations of this classic "cell membrane receptor-centric" paradigm have gradually become apparent: it struggles to fully explain why ketamine can take effect rapidly within hours of administration, why its efficacy can last for weeks, and why there are significant differences in efficacy and side effects between its different enantiomers. More importantly, an understanding confined solely to the level of cell membrane receptors offers limited systematic guidance for designing new molecules with potential for improvement.
Notably, as a typical psychoactive substance, after ketamine crosses the blood-brain barrier and enters the central nervous system, it not only acts on various membrane protein targets but also inevitably distributes widely within the intracellular environment of neurons and glial cells. However, the understanding of its intracellular targets and molecular events has long remained relatively obscure, with most research still focusing on cell membrane receptors such as ion channels, GPCRs, or transporters.
Against this backdrop, the team led by Wang Xiaohui at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, has taken a unique approach in recent years. Using chemical biology techniques as their methodological core, they have systematically integrated three main lines of inquiry—"intracellular target identification," "cellular metabolic analysis," and "synthetic chemistry and structure-activity relationship studies"—gradually building a mechanistic model that more closely reflects the true picture inside cells. This provides a new perspective beyond the traditional cell membrane receptor paradigm for comprehensively understanding ketamine's rapid and sustained antidepressant effects.
The starting point of their research was a "previously overlooked enzyme." The team ingeniously used active group probes and proteomic pull-down techniques to successfully "fish out" deacetylase SIRT2 from the complex cellular protein network—a target previously rarely associated with ketamine (Adv. Sci. 2025, 12, e2416481). Subsequent cellular endogenous CETSA thermal stability assays and fluorescence titration experiments consistently confirmed that S-ketamine can bind directly to SIRT2, while the binding capacity of R-ketamine is minimal. This discovery not only quantitatively clarified its enantioselectivity but also provided key molecular evidence to explain the differences in anti-inflammatory and antidepressant effects between its enantiomers. Further molecular dynamics simulations revealed a key player in the binding process: residue Q167. In 200-nanosecond simulations, S-ketamine maintained stable binding through the formation of hydrogen bonds and hydrophobic interactions with Q167, whereas R-ketamine dissociated in the later stages of the simulation. Subsequent site-directed mutagenesis experiments showed that mutating Q167 to alanine significantly weakened the binding strength of S-ketamine, a computational result corroborated by the observed decrease in protein stability via CETSA.
Binding evidence alone is insufficient to fully elucidate the mechanism of its pharmacological effects. Therefore, using two ketamine probes with different design concepts, the team systematically conducted enrichment, silver staining, LC-MS/MS identification, and immunological validation in tissues and microglial cells. Repeated experiments consistently identified SIRT2 as a common and reproducible target protein. Stern-Volmer fluorescence titration and CETSA data within the same system mutually supported each other, providing methodological self-consistent verification for the "direct binding–functional effect" causal chain. At the cellular phenotype level, the study further quantified the activation state of microglia in the medial prefrontal cortex and the transcription levels of inflammatory factors TNF-α/IL-1β. They found that S-ketamine treatment effectively suppressed neuroinflammation, an effect direction highly consistent with the intrinsic logic of "activating deacetylase–inhibiting inflammation" (Fig. 1). Through RNA interference, in vitro intervention with the SIRT2 inhibitor AK-7, and in vivo pharmacological blockade experiments, it was further confirmed that SIRT2 is a key mediator of ketamine's anti-inflammatory and antidepressant effects. Notably, depression is highly comorbid with chronic neuroinflammation, and this study suggests that the anti-neuroinflammatory effect mediated by ketamine via SIRT2 may be one important mechanism for its long-lasting antidepressant efficacy. These findings not only establish the SIRT2-NF-κB signaling axis as a central hub for ketamine's reversal of depression-related inflammatory phenotypes but also provide a theoretical and experimental basis for developing targeted therapeutic strategies for neuroinflammatory depression.
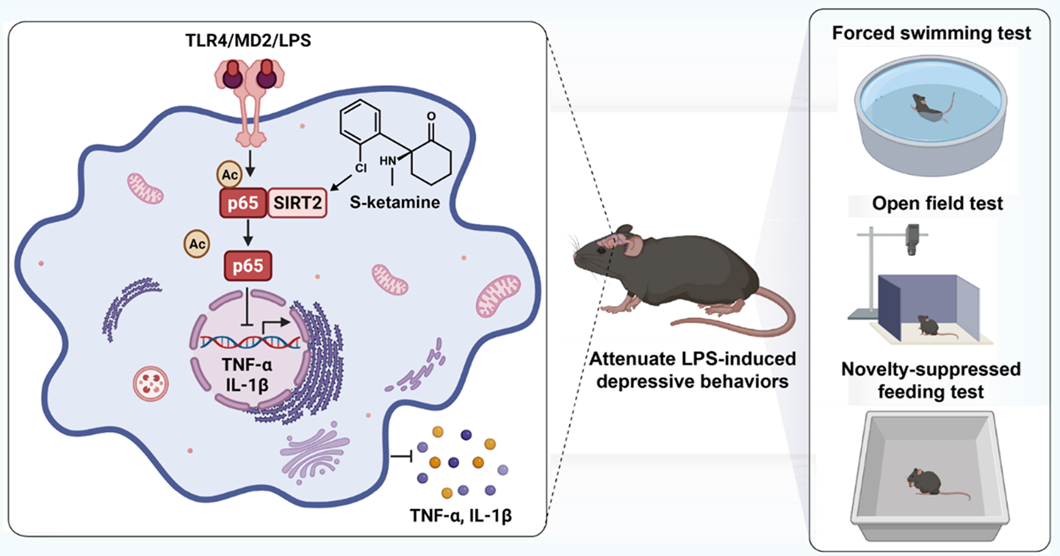
Fig. 1. S-ketamine alleviates neuroinflammation and depressive-like behaviors by directly binding the intracellular target SIRT2.Image by Wang Xiaohui
Repeated target fishing using different ketamine probes has revealed their interactions with proteins associated with mitochondrial energy metabolism (Adv. Sci. 2025, 12, e2416481). Luo Minmin's team identified adenosine as a key messenger mediating ketamine's rapid antidepressant effects, while Wang Xiaohui's team systematically modified the ketamine structure, focusing on the chlorine substituent on the aromatic ring, the sixth position of the cyclohexanone ring, and the amino side chain. Collaborative research between the two teams revealed that dechloroketone derivatives—particularly dechloroketamine (DCK) and dechloro-N-ethylketamine (2C-DCK)—induced significantly stronger adenosine release than ketamine at equivalent doses. Among them, DCK demonstrated excellent antidepressant efficacy at extremely low doses, alongside a significant reduction in dissociative side effects (Fig. 2). Most notably, a clear decoupling was observed between adenosine release capacity and NMDA receptor inhibition, suggesting that ketamine’s antidepressant action is not entirely dependent on NMDA receptor blockade (Nature, 2025, DOI: 10.1038/s41586-025-09755-9).
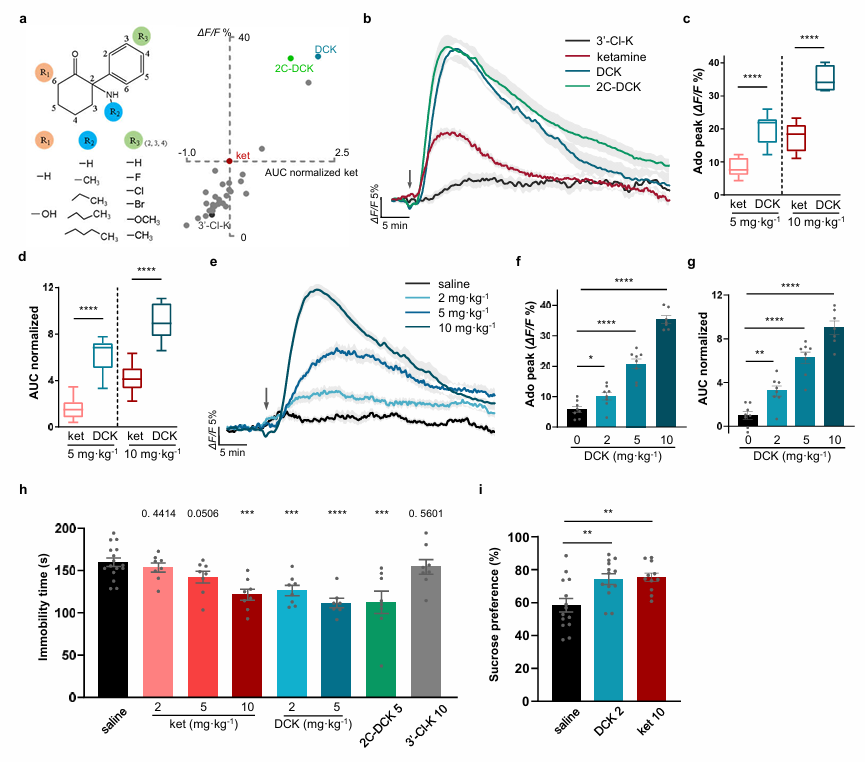
Fig. 2. Screening of ketamine analogs based on adenosine signaling and validation of their antidepressant effects. (a), Schematic diagram of ketamine analog design and in vivo adenosine release screening. Left: Compound structure modification strategy; Right: Scatter plot of peak adenosine release and area under the curve (AUC) induced by various compounds after in vivo administration (10 mg·kg⁻¹), data normalized to ketamine response. (b), Time course of extracellular adenosine dynamics in the medial prefrontal cortex after injection of DCK, 2C-DCK, 3′-Cl-ketamine, and ketamine (10 mg·kg⁻¹). Black arrows indicate administration time points. (c, d), Comparison of peak adenosine release (c) and AUC (d, normalized to saline group) induced by DCK and ketamine at equivalent doses. (e–g), Dose-effect analysis of DCK: Time course (e), peak (f), and AUC (g) of adenosine release after injection of different doses of DCK (2, 5, 10 mg·kg⁻¹) or saline. (h, i), Rapid antidepressant-like behavioral effects of preferred compounds in the chronic restraint stress model: immobility time in the forced swim test (h) and preference percentage in the sucrose preference test (i), measured 1 hour after intraperitoneal injection. Tested doses for ketamine and DCK are indicated; 2C-DCK used 5 mg·kg⁻¹, 3′-Cl-ketamine used 10 mg·kg⁻¹.Image by Wang Xiaohui
To support the in-depth study of the structure-activity relationships mentioned above, Wang Xiaohui's team developed a concise and efficient α-bromination synthesis route, constructing the key 2-bromo-2-aryl-cyclohexanone intermediate in one step under mild conditions using an NBS-DMSO-CHCl₃ system. This route exhibits excellent substrate applicability and scaling potential. Compared to traditional free radical pathways, three-step epoxidation–ring opening–oxidation routes, and acidic bromination methods, it demonstrates superior regioselectivity and operational convenience, and has been validated at the 15 mmol scale, laying a synthetic foundation for the rapid construction of ketamine-like derivatives (Org. Biomol. Chem., 2025, 23, 1627-1632). Furthermore, through fine-tuning of temperature and steric factors, the team achieved controllable switching between the competing pathways of nucleophilic substitution and the Favorskii rearrangement: at -25°C and lower temperatures, primary amines predominantly led to the nucleophilic substitution pathway, efficiently generating ketamine analogs; whereas under increased temperature, use of secondary amines, or with ortho-substituted aryl rings, the reaction favored proceeding through the Favorskii rearrangement to construct the 2-aryl-cyclohexanone-1-carboxamide skeleton. This "low-temperature substitution–high-temperature rearrangement" rule was validated through substrate scope exploration and theoretical calculations, forming a programmable, scalable synthetic strategy that powerfully facilitates the efficient discovery and optimization of ketamine-like lead molecules (Org. Biomol. Chem., 2025, 23, 2704-2711).
The core insight from this series of works is that the intracellular pharmacology of psychoactive substances should be regarded as a dimension equally important as the study of cell membrane receptors and neural circuits. The research elevates dynamic processes like "metabolism–adenosine" from phenomenological footnotes to quantifiable, intervenable mechanistic readouts. Simultaneously, it deeply embeds synthetic methodology into the research pipeline, enabling efficient, closed-loop iteration between structure-activity relationship studies and mechanism validation. Consequently, the vision for "faster, more stable, and safer" ketamine-derived therapeutic strategies is no longer just an aspiration but possesses a clear technical pathway and actionable leverage points. More importantly, ketamine is being redefined: it is not merely a molecule primarily acting on membrane receptors, summarized as a "potent synaptic plasticity promoter," but rather a systemic pharmacological modulator capable of triggering precise cascading reactions at the intracellular and energy metabolism levels. This paradigm provides a replicable, accelerable, and extensible general methodology for subsequent research and development.
