Sandwich complexes in which a metal center is bound by two arene ligands have captured the imagination of chemists ever since the first example, ferrocene, Fe(C5H5)2, was reported in 1951. Besides being cool molecules, a variety of these “metallocene” compounds of different shapes, sizes, and compositions have found applications in catalysis and as building blocks to make new chemicals and materials. A research team in China has now introduced a new item on the molecular sandwich menu: the first all-metal aromatic sandwich complex, [Au3(Sb3)2]3–. The triple-layered prismatic anion consists of a triangular Au3 cluster between two triangular Sb3 clusters (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b07730). Hua-Jin Zhai of Shanxi University, Zhong-Ming Sun of the Chinese Academy of Sciences’ Changchun Institute of Applied Chemistry, and coworkers made the complex by treating a gold phosphine compound with an ethylenediamine solution of K5Sb4 containing the chelating agent 2,2,2-cryptand. The team found that the new sandwich, isolated as a potassium cryptand salt, is held together by Au-Sb σ bonds and that the antimony rings have π aromatic character. “We believe the complex should pave the way for syntheses of new classes of sandwich compounds and may find technological applications, such as in low-temperature nanogold catalysis,” Zhai says. (C&EN)
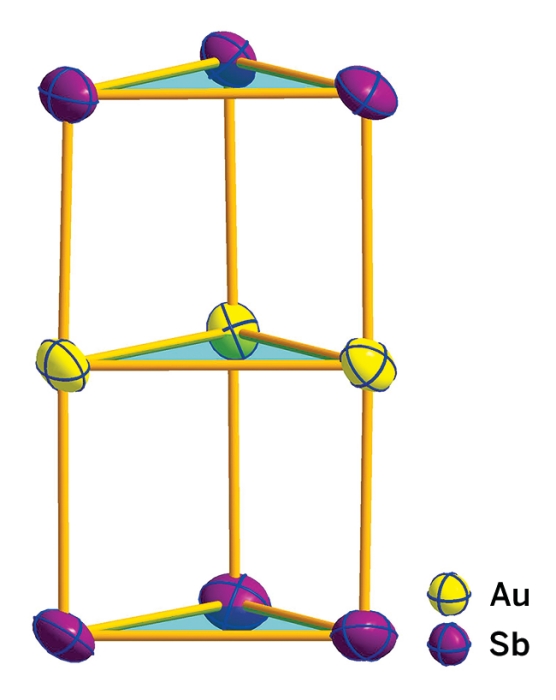
Image by ZHAI Huajin
Contact:
SUN Zhong ming
Email: szm@ciac.ac.cn
State Key Laboratory of Rare Earth Resources Utilization, Changchun Institute of Applied Chemistry
